While the phrase "Electronic Data Capture" (abbreviated EDC) may sound pretty general, in the context of clinical trials, it really refers to the use of technology to gather clinical trial data electronically rather than on paper.
What is an electronic data capture system?
Most contemporary electronic data-capturing software runs on the web and makes use of a thin client. Web-based software operates fully on a Web server; see Google.com. Thin client software simply requires a standard web browser that is connected to the internet in order to access and use the EDC system. No complicated plug-ins are required.
Clinical trial data can be recorded on paper and then transcribed into the EDC system, or it can be recorded electronically at the source (referred to as a "e-source").
What is electronic data capture used for?
Most clinical studies that are started now employ computerized data-gathering technologies. Users of EDC software fall into three main categories: sponsors, CROs, and sites.
- Sites: A site is a facility, often a hospital or clinic, that arranges and gathers data from clinical trial volunteers, or patients. Data entry into the research's Electronic Data Capture (EDC) system is usually assigned to nurses or other designated study "coordinators" who work for the site. The doctor in charge of the patient's care and data at the site, known as the Investigator, is in responsibility of checking and digitally signing the data.
- Sponsor: An entity that "owns" a clinical study is its sponsor. Before releasing a product into the market, biopharma, device, and other life sciences businesses must fund clinical studies to obtain regulatory bodies (such as the FDA) to approve their medical breakthroughs. Different persons who utilize the EDC system in different positions can be employed by sponsors.
In order to check the correctness of related data in the EDC system, monitors acting on behalf of the sponsor may visit the client locations to evaluate data source documents (with EDC software, this "visit" is commonly virtual). Biostatisticians assist with planning and data analysis. Data managers are usually significant users of EDC tools, and part of their job is to make sure the trial data is clean and useable. They might, among other things, send "queries"—requests for information—to the sites in order to get data problems clarified and fixed.
- CROs: Contract research organizations, or CROs, are companies that enter into agreements with sponsors to help with the organizing and execution of clinical trials. In certain cases, the CROs may manage the study efficiently on behalf of the sponsor. They will only assume a portion of the major responsibilities in other trials (data management, monitoring, analysis). In this sense, sponsors and CROs may have many of the same kinds of EDC system users.
CROs are sometimes referred to as Clinical Trials Units, Data Coordinating Centers, or AROs (Academic Research Organizations) in the academic community. They perform managerial and coordination duties that are mostly similar to those of their commercial counterparts.
Study participants may additionally provide data to the EDC system in addition to the aforementioned user categories. This can be done directly through a dedicated role in the software or indirectly through a different device and/or application that sends data to the EDC system. Electronic patient reported outcomes, or ePRO, is the process by which patients submit data.
Why use electronic data capture software?
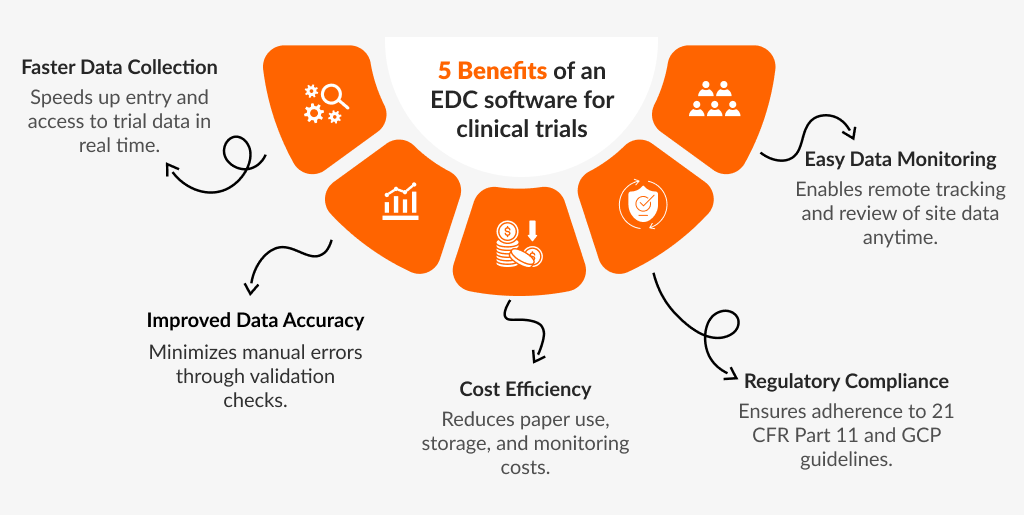
Today, computerized data gathering software is used in most new clinical studies. Common reasons for utilizing EDC include the following:
- Cleaner Data: EDC software excels at enforcing certain data quality requirements. Before data is allowed into the trial database, edit checks and source data verification included into the software may ensure that the data matches the needed formats, ranges, etc.
- Efficient Procedures: EDC software can assist in guiding the site through the sequence of research events by simply seeking the information required at a given moment for the specific patient's situation. It can assist in decreasing the number of in-person site visits necessary for a trial and facilitate the process of resolving data inconsistencies using tools for finding and fixing data problems with sites.
- Faster Data Access: In a clinical study, web-based EDC solutions can offer almost instantaneous data access. This realization facilitates quicker decision-making and may help with adaptable trial designs.
Because they all deal with productivity and efficiency concerns, the aforementioned justifications for adopting EDC can help lower the cost of clinical trials.
What is the difference between EDC and eCRF?
EDC and eCRF are not the same thing. Software called electronic data capture, or EDC, is used to gather data from clinical trials. An electronic case report form, or eCRF, is a type of digital questionnaire used to gather information on research participants. It is often web-based. Clinical data managers and other clinical research stakeholders may gather better data more quickly thanks to eCRFs, which are housed inside the EDC.
How do EDCs support data management?
EDC solutions facilitate data management by providing a simplified, adaptable method of gathering pertinent patient data points. The specific needs of the user will determine what data is gathered and submitted. The majority of systems let you choose what kinds of data points to gather. Frequently gathered data comprises:
- Participant demographics in clinical trials
- Thorough medical histories that include past illnesses, treatments received in the past, surgeries, prescription drugs, and allergies
- Clinical evaluation information, such as test findings, vital signs, and physical examination results
- Safety information that records patient responses or side effects during the study, as well as adverse events (AE)
- Patient-reported results, such as pain measures, quality of life evaluations, and comparable subjective insights
Access to the sensitive clinical trial data that’s collected can be limited to only authorized individuals using most EDC systems. Authorized personnel may include researchers, clinicians, data managers or study coordinators. Remote viewing of EDC data for authorized users is a feature that can make centralized data management possible for multi-site studies.
The benefits of an EDC system
Clinical trial data management and gathering are streamlined by electronic data capture (EDC) technologies. More validation checks guarantee correct data, and instantaneous data availability facilitates quicker decision-making. More precisely, they encourage success by providing:
- Precision- Because EDC systems include integrated management capabilities that support accuracy, they help to improve the quality of the data. On data entry forms, constraints can be imposed to stop users from entering erroneous or nonsensical values. Cleaner, more dependable data is produced via integrated error detection systems, which also enable automatic computations and solve problems with illegible submissions.
- Compliance- Sensitive patient data is kept secure thanks to strong data security procedures. These technologies also make it easier to incorporate limitations and validation tests that comply with legal standards. Most systems have the ability to trace changes to data and user activity in detail, which helps with regulatory compliance and streamlines audit procedures.
- Security- It is much simpler to launch a product when every adverse event (AE) can be reviewed to see if it fits the requirements for endpoint adjudication. Ensuring effective collection, storage, and management of data streamlines the process as a whole.
In Summation
Better features lead to an enhanced user experience and better UX leads to enhanced productivity and efficiency. This in turn results in better data and thus a better study. But our spirit of consistent innovation doesn’t stop with our EDC. As a core component of our eClinical suite Octalsoft's EDC is simply the starting point for revolutionizing clinical data management.
Choosing the appropriate EDC software is critical to the success of your clinical research projects. While the market has various solutions, Octalsoft's EDC stands out due to its unparalleled scalability, steadfast dedication to compliance, user-centric design, easy integration possibilities, and reasonable price. Its comprehensive features and dedicated user assistance make a compelling value proposition, making it the chosen choice for researchers looking to optimize clinical trials and fulfill their research objectives more successfully.
However not every EDC can match the requirements of a modern clinical trial. Octalsoft’s EDC solution, is your one-stop shop for all your data capture software needs. Interested in knowing how Octalsoft’s EDC can streamline your clinical trial data and ensure 10X accuracy?