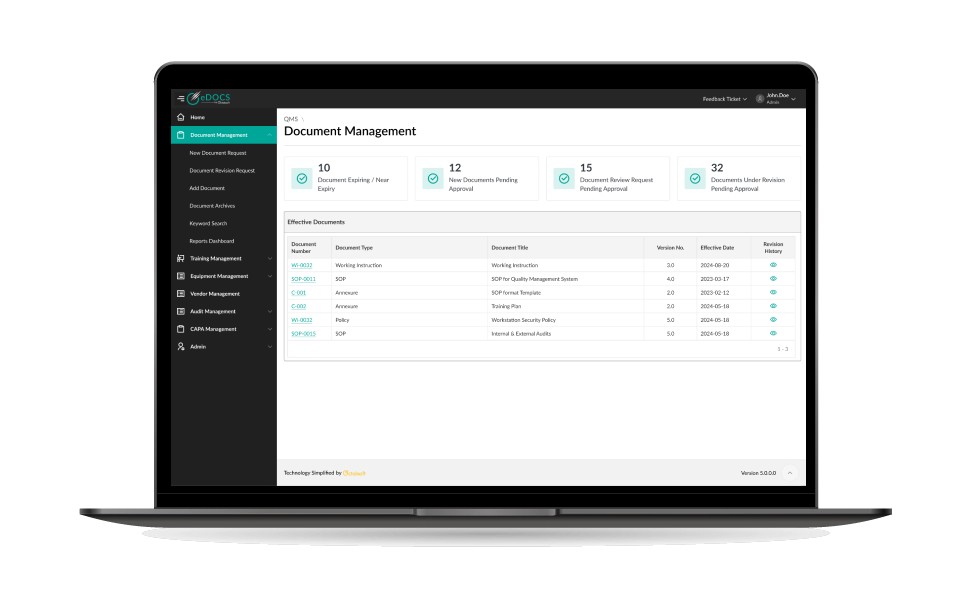
Product Features
Instant Document Availability
Octalsoft eDOCS ensures real-time availability of most up to date, approved and current content, making it accessible wherever you may be in the world. It makes sure that the user has access to the most recent document without the worry that they are accessing an outdated version.

Centralized Document Repository
The software provides a centralized data repository for storing and managing your essential trial documents with robust e-tools for collaboration, version control, CAPA, training, and reporting. A centralized repository with study level correspondence and documentation eliminates inefficiencies.

Easy Document Review
With real-time access, the software enables easy review, annotation or publishing of document updates with all partners including internal and external cross-collaborators. It makes alliances with third-party organizations and research partners much easier. The level of visibility and access are determined through controlled access.

Version Control
The software allows for complete version control and history management of obsolete versions of documents. It provides a complete audit trail of who made the changes to the content and when. It also tracks all edit/review/approval routing including those performed by multi-user collaboration.
Instant Document Availability
- Near real-time availability of most up to date, approved and current content
- Un-approved documents are not accessible to end-users
- Centralized data repository for storing and managing your essential trial documents

Easy Document Review
- Robust e-tools for collaboration, version control, CAPA, training, and reporting
- Easy review, annotation or publishing of document updates with all partners including internal and external cross-collaborators
- Complete version control and history management of obsolete versions of documents
- Tracks all edit/review/approval routing including those performed by multi-user collaboration

Our Vetted Experience
Related Solutions

CTMS
Maintain a centralized, relevant, and most up to date study and operational database; thus providing users with total control, while complying with all regulations.

EDC
Accelerate the speed of your clinical trial by reducing deployment time, capturing clean data quicker, timely study close-out and early data lock efficiently.

IWRS
Effectively configure subject enrolment and randomization process and also manage global IP supply chains, using an intuitive web-browser interface.