Product Features
Intuitive User Interface (UI)
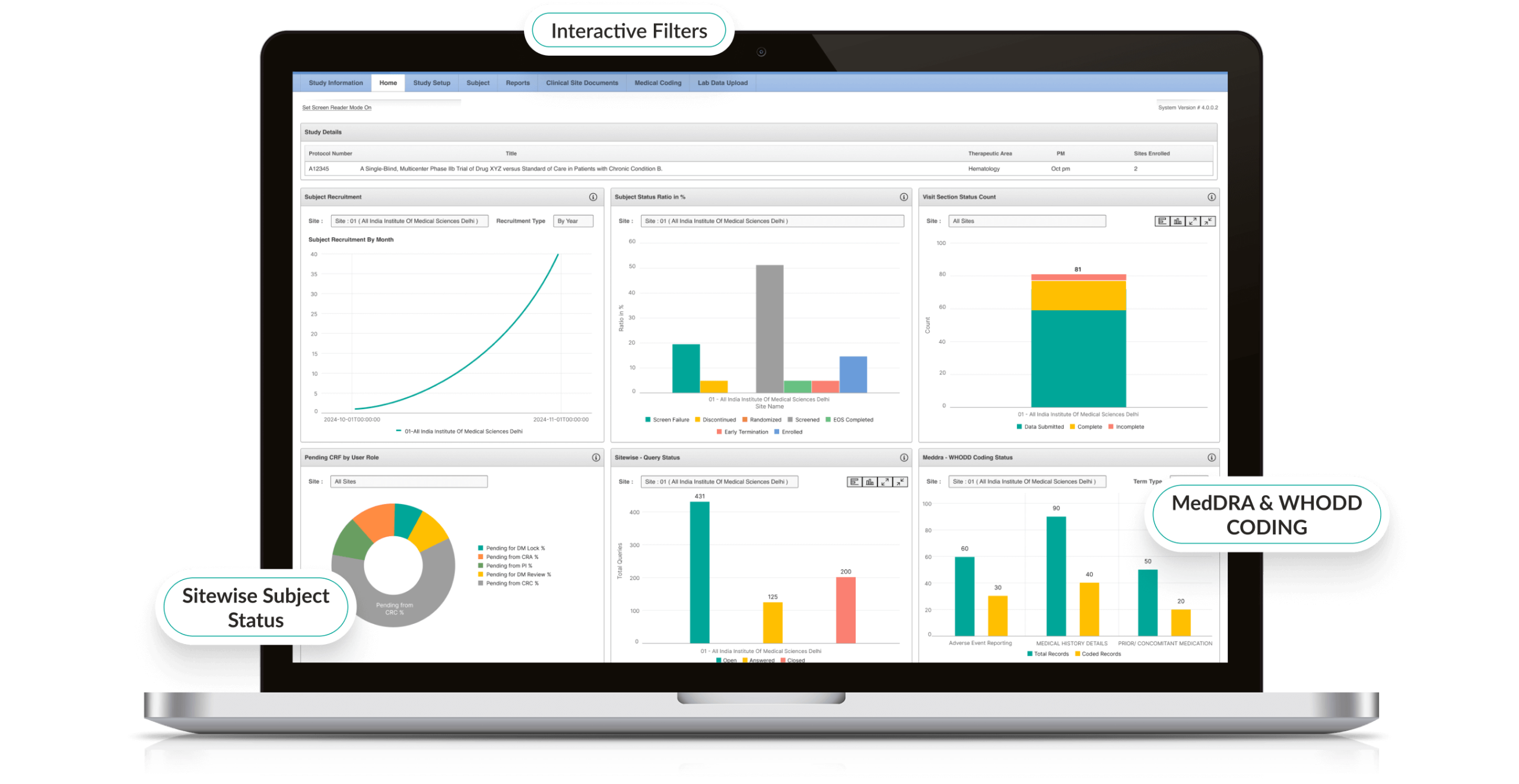
Octalsoft EDC’s UI, with easy navigation, is intuitive enough even for new users to get the hang of it quickly. Simple page layouts, dynamic questions, chronological response mechanisms, responsive autocomplete fields, conspicuous progress bars, all make a significant difference.

Study Planner
Robust study planner with unlimited data fields enables setting up complex calculations and field dependencies in a matter of weeks. It allows cloning and reuse of study components across trials and simplifies study configuration.

eCRF Designer
Octalsoft EDC’s friendly eCRF form builder allows you to efficiently build and manage multiple eCRFs across various trial phases. The system helps you design your eCRFs and even make mid study changes with a simple drag-and-drop interface, without the need of any IT support.

Online Data Validation
Real-time edit checks at the point of data entry ensure that the data generated is clean and accurate. The system re-orients the user when their input conflicts with pre-defined field requirements. Various system-level checks quickly identify transcription errors, missing and duplicate data.

Query Management
Streamlined communication between monitors, data managers, and study coordinators ensure issues with data discrepancies are addressed promptly. Our system not only comes with the capability to manually add queries but also auto-generate queries based on logic validation.
Quicker Access to Data
- Quick and clear overview of patient data at all times
- Robust data validation checks and automatic calculations
- Generate compliant, near submission-ready data; thus, facilitating faster submission of study results for regulatory approval.

Compliance and Standardization
- Compliant with regulatory requirements like 21 CFR Part 11 and HIPAA Privacy Act
- Maintain a complete audit trail of clinical trial data generated
- Protect the confidentiality of subjects
- Adheres to independent industry standards like CDISC

Our Vetted Experience
Downloads
Related Solutions

CTMS
Maintain a centralized, relevant, and most up to date study and operational database; thus providing users with total control, while complying with all regulations.

IWRS
Effectively configure subject enrolment and randomization process and also manage global IP supply chains, using an intuitive web-browser interface.

eTMF
Deploy a highly effective eTMF solution to electronically capture, organize, share, and store all those essential trial documents, images, and artifacts.