Product Features
Study Setup and Configuration
Streamline the setup of clinical trials, including defining study protocols, eligibility criteria, and randomization processes. Our eClinical Analytics offers end-to-end trial management capabilities, from study setup and patient recruitment to data collection and analysis.

Patient Recruitment and Management
Identify eligible patients, manage enrollment, and track their progress throughout the trial. We believe that technology should be accessible to everyone. Our user-friendly interface is designed with simplicity in mind, making it easy for both professionals and newcomers.

Electronic Data Capture
Collect patient data electronically, reducing errors and enhancing data quality. Stay informed with real-time access to critical trial data. Monitor progress, track patient participation, and make informed decisions on the fly, improving the efficiency of your trials.

Data Analysis and Reporting
Generate insightful reports and analytics to aid in decision-making and data-driven insights. We understand the importance of data security and compliance in healthcare. Octalsoft's eClinical Analytics adheres to industry-leading security standards and complies with all relevant regulations.

Integration Capabilities
Seamlessly integrate with other clinical trial systems and databases for a unified experience. Every clinical trial is unique, and your technology solutions should reflect that. Our dashboard is highly customizable to adapt to your specific needs and can scale as your research projects grow.
Data Centralization
- Centralize various data sources, providing a single location for clinical trial data
- Researchers and stakeholders can access near-real-time data
- Data visualization tools that help users quickly understand and interpret complex clinical trial data, trends, and patterns
- Create customized reports and metrics tailored to specific research needs.

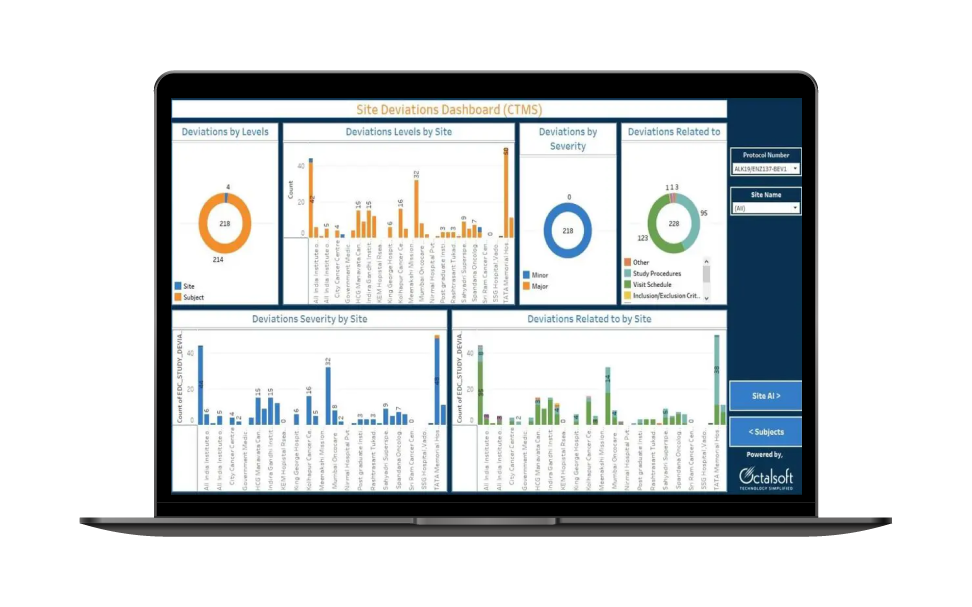
Efficient Site Mangement
- Monitor clinical trials remotely, reducing the need for physical site visits and improving efficiency
- Track protocol adherence, ensuring that the trial is conducted in accordance with the study plan
- Manage and coordinate activities at multiple trial sites more efficiently

Our Vetted Experience
Related Solutions

CTMS
Maintain a centralized, relevant, and most up to date study and operational database; thus providing users with total control, while complying with all regulations.

EDC
Accelerate the speed of your clinical trial by reducing deployment time, capturing clean data quicker, timely study close-out and early data lock efficiently.

IWRS
Effectively configure subject enrolment and randomization process and also manage global IP supply chains, using an intuitive web-browser interface.