Our Vetted Experience

Scale with Simplicity
Octalsoft IWRS has a simple and effortless UI that speeds up site adoption, reducing the learning curve for site staff, including coordinators and investigators. With intuitive navigation and clear workflows,the system prevents mistakes during critical tasks like subject enrollment or IP allocation. A simple UI/UX adapts easily to trials of varying complexity (Phase I-IV) or size, ensuring consistency for multi-center or global clinical trials.

Flexible Randomization Algorithms
Octalsoft IWRS empowers clinical trials with versatile randomization methods—simple, block, stratified, adaptive, and minimization—customized to each study’s demands. For intricate designs, it seamlessly manages multi-arm, multi-stage, or response-adaptive protocols, delivering accurate patient assignments to treatment groups with precision.

Optimize IP Supply with Predictive Algorithms
Octalsoft IWRS optimizes resupply by automating orders based on real-time site consumption and trial milestones, minimizing manual effort and enhancing logistics efficiency. Its advanced predictive analytics calculate optimal buffer stock, factoring in trial complexity, site variability, and randomization schedules, fine-tuning overages to curb excess IP while protecting against sudden demand surges.

Blinding and Allocation Concealment
Octalsoft IWRS embeds advanced blinding protocols to protect randomization schedules and treatment arm assignments, seamlessly enforcing single, double, or triple blinding. Leveraging granular role-based access controls and tamper-proof audit trails, it ensures robust allocation concealment, upholding trial integrity from start to finish.

Break the Code-Not the Trial
Octalsoft IWRS swaps paper envelopes for a secure digital code break system, streamlining emergency and non-emergency unblinding. Real-time authorization workflows empower designated users to unblind seamlessly, preserving trial blinding integrity. Built-in alerts and reports deliver instant visibility to clinical trial sponsors and CROs, ensuring robust oversight and global regulatory compliance.

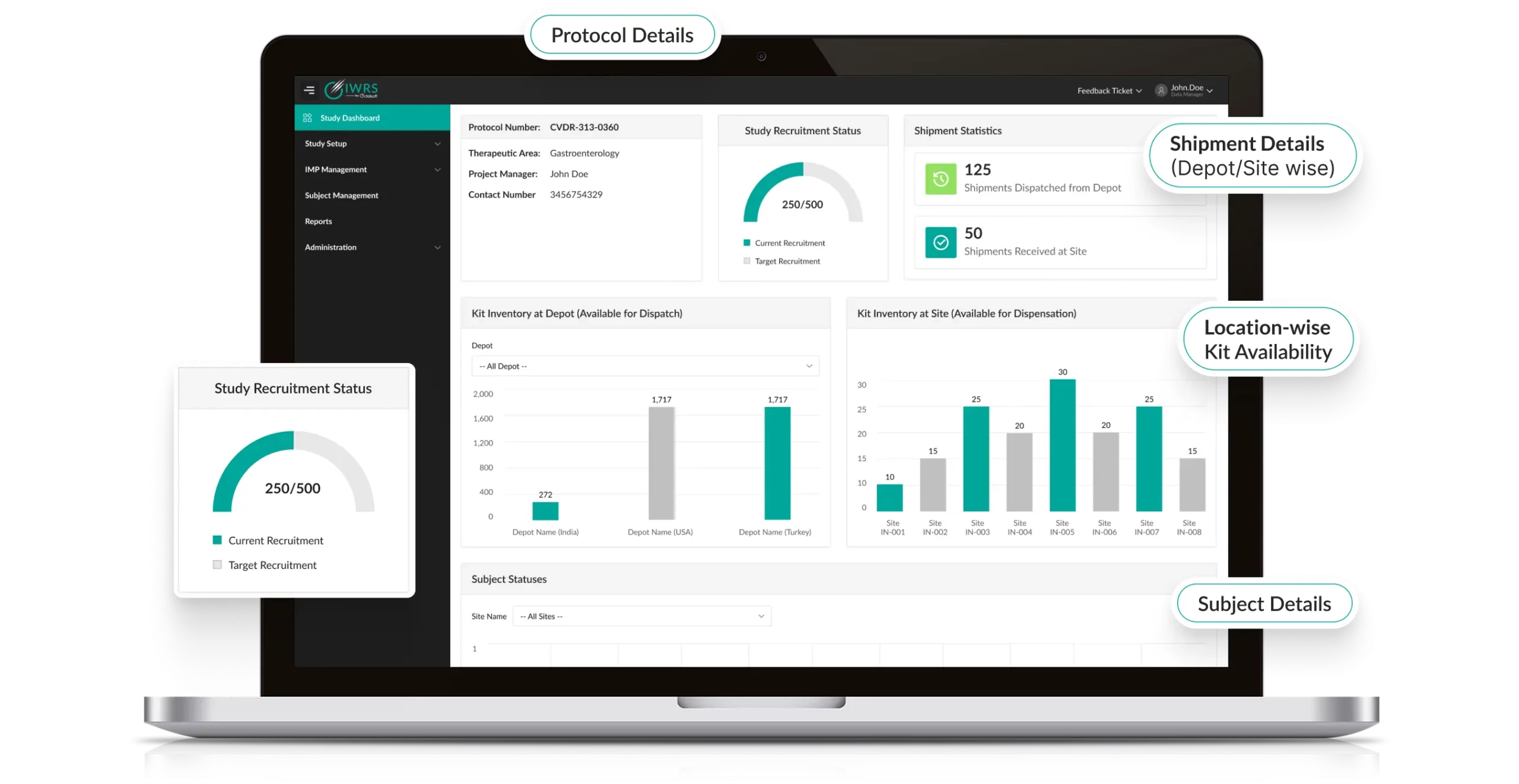
Track it LIVE-From Start to Finish
Octalsoft IWRS delivers real-time, customizable notifications for critical events such as low stock, delayed shipments, or temperature deviations, enabling proactive IP replenishment management. Built-in reports deliver automated, customized analytics, such as subject progress and IMP usage, empowering data-driven decision-making.
Seamless Recruitment, Budget-Friendly Trials
Octalsoft IWRS delivers a robust, web-based platform for clinical trials, enabling 24/7 access to streamline subject enrollment, randomization, and IMP dispensing. By integrating advanced randomization algorithms and real-time inventory tracking, the system optimizes trial workflows, minimizes delays, and reduces operational costs.
Designed for rapid deployment, it supports complex study designs, ensuring precise cohort allocation and accelerated time-to-market for novel therapies.
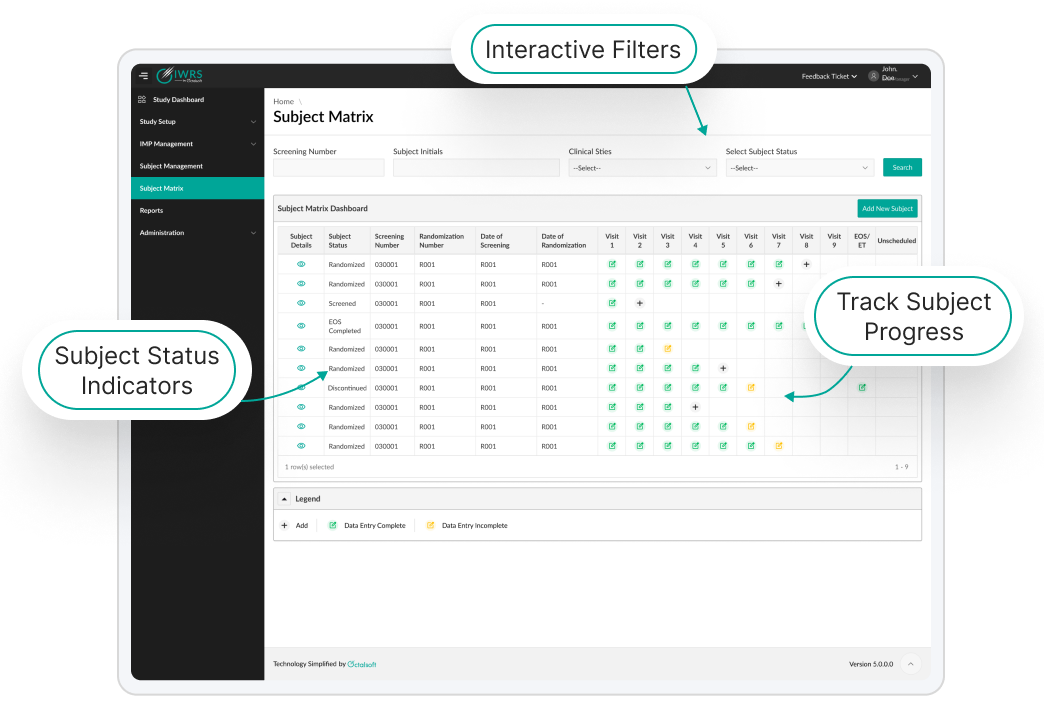
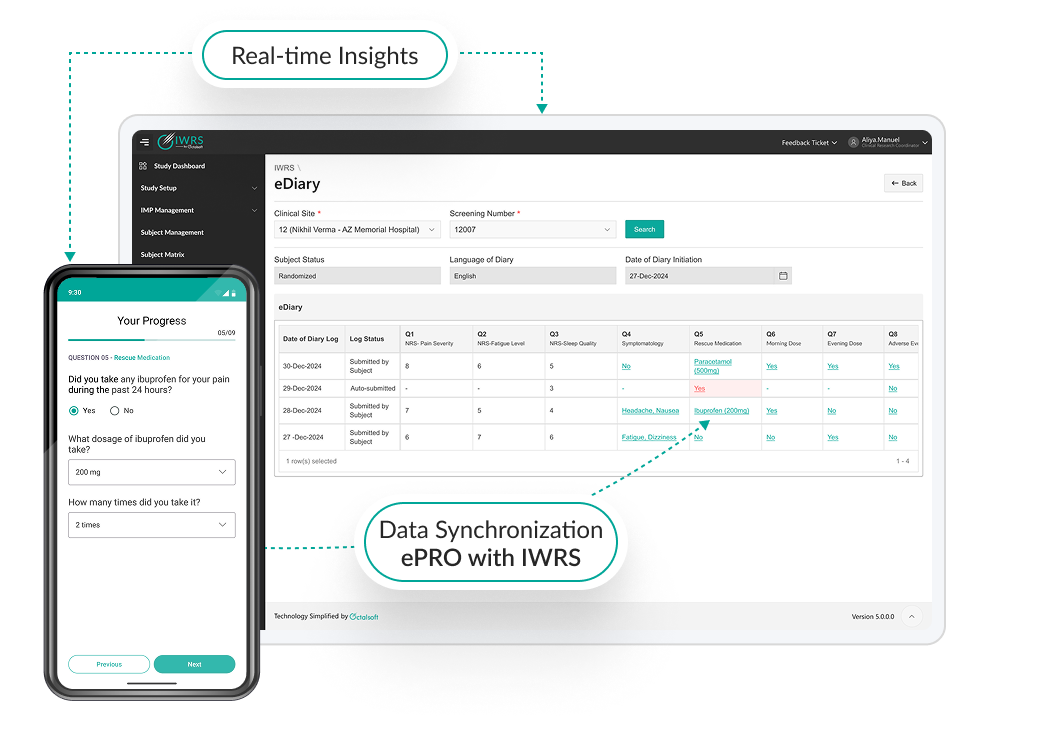
Unified Ecosystem for Enhanced Trial Integrity
Octalsoft IWRS ensures fluid integration with its native solutions and third-party clinical platforms, such as Electronic Data Capture (EDC), Electronic Patient-Reported Outcomes (ePRO), and Clinical Trial Management Systems (CTMS).
This robust interoperability facilitates real-time data synchronization across all trial processes, optimizing workflow efficiency, enhancing data accuracy, and reducing manual reconciliation efforts.
Our Vetted Experience
Downloads
Related Solutions

CTMS
Maintain a centralized, relevant, and most up to date study and operational database; thus providing users with total control, while complying with all regulations.

EDC
Accelerate the speed of your clinical trial by reducing deployment time, capturing clean data quicker, timely study close-out and early data lock efficiently.

eTMF
Deploy a highly effective eTMF solution to electronically capture, organize, share, and store all those essential trial documents, images, and artifacts.