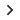Using information from earlier studies, risk-based monitoring (RBM) is a clinical trial strategy that estimates the likelihood that a patient may have certain adverse effects. By identifying and reducing hazards at the outset of the research and continuing to assess them over the course of the investigation, this approach contributes to patient safety. Furthermore, RBM conducts in-depth research on every facet of study design, such as:
- Protocol creation
- Research methodology
- Locations for clinical research (sites)
With this strategy, sponsors may see prospective problems early and take remedial action before missing data or inadequate reporting of patient events compromises patient safety, data integrity, or quality control.
What is Risk-Based Monitoring Technology?
The goal of risk-based monitoring software, or RBM, is to detect concerns early in a research and make sure they are adequately addressed before they develop into significant problems. In order to do this, RBM examines a few crucial traits, including gender, age group, and health status, of individuals who have previously been enrolled in clinical trials. Based on these attributes, the findings are then sorted together into cohorts (or groups).
Sponsors of new drug development programs may examine the data gathered from each research and, more swiftly than ever before, forecast how people will respond to novel drugs or experimental therapies under evaluation.
Additionally, RBM solutions gives sponsors the chance to increase openness by enabling better real-time reporting of adverse events (AEs) when monitors enter patient data into the system.
RBM is a proactive approach to clinical trials in this way. RBM entails continuous trial monitoring as opposed to traditional monitoring, which only recognizes issues when they arise and then takes appropriate action. A more thorough strategy is needed for the successful administration of today's large-scale, long-term clinical trials, although traditional monitoring techniques may have worked well 20 or 30 years ago when clinical studies were small and had a limited duration.
Core and Optional Activities
The RBM method allows for the usage of both core and optional activity categories. While optional activities are not necessary but can be selected as part of a personalized RBM approach, core activities are a must for all clinical trials.
Core/Optional Activities:
RBM, or risk-based monitoring, is a fundamental task. This activity entails developing a strategy to track risk variables, such as protocol deviations or adverse events (AEs), in order to spot any changes that could have an impact on patient safety or study conduct.
Although they are not necessarily mandated by regulations, Data Management Plans (DMPs) are an optional activity that sponsors frequently employ to expedite their data gathering process and ensure that all procedures have been followed appropriately during the research period.
Benefits of Risk-Based Monitoring Tools in Clinical Trials and RBM Process
Risk-based monitoring enhances the quality, efficiency, and adherence to good clinical practice (GCP) of clinical trials by employing a methodical methodology to monitor patient safety and clinical data quality.
Benefits of RBM in respect to the overall trial conduct include:
- Put patient safety first by spotting issues early on so they may be fixed before more significant harm happens. This lessens the chance of bias or error compromising the integrity of your trial data.
- Use resources more effectively by concentrating on regions with a higher chance of mistakes or issues rather than allocating the same amount of effort to each area.
- Aid in lowering the total expense of conducting clinical studies.
- Instead of creating many reports for each activity, create a single report to facilitate more effective monitoring.
- Streamline workflows by automating repetitive tasks like filling out forms and entering data into computers.
- Enhance the patient experience by using mobile devices or online portals to gather data in real-time from any place at any time.
Risk-based monitoring has various advantages for both the patient and the sponsor when used in clinical studies.
Risk-based monitoring has various advantages for both the patient and the sponsor when used in clinical studies. The main advantage is in lowering the total number of patients exposed to the research medication, which lowers expenses at every stage of the clinical trial's life cycle, from planning and development to production and distribution.
This is a crucial factor to take into account because many sponsors have extremely tight budgets for creating new medications. Reducing needless exposure will also make it more likely that all participants will have access to rare medicines should they become essential during or after the study.
Sponsors are encouraged by risk-based monitoring to carefully consider whether their product should be tested on individuals with varying degrees of health concerns related to its use. Furthermore, depending on the product and protocol design, RBM assists sponsors in determining if this kind of monitoring should take place during all trial stages or only certain phases, such as preclinical research prior to human trials.
Conclusion
We've spoken about the definition of risk-based monitoring in clinical trials and its significance. Risk-based monitoring technology can help you stay on track with your trial goals and is an excellent way to save expenses and boost efficiency for your clinical trial. It gives you the ability to keep an eye on just the most important metrics, which makes it an affordable way to enhance quality control and make sure you don't go over budget.
Consequently, RBM is a valuable tool for sponsors and patients alike, who want safe and economical research undertaken.
Ready to optimize your monitoring strategy and drive improved outcomes? Click here to request a demo and experience the transformative power of the OctalSoft eclinical suite and its Risk-Based Monitoring capabilities.